Table of Contents
Introduction
The Molecular Structure STEM Kit is an innovative learning tool designed specifically for students in grades 7 through 9 . This kit aims to transform the abstract concept of molecular structures into a tangible and interactive experience. By providing students with hands-on activities, the kit helps bridge the gap between theoretical knowledge and real-world application, making the study of chemistry more engaging and accessible.

Objective
Machines and Materials Required
- 3D Printer (Creality K1C)
- Slicer software (Creality Print)
- Colored filaments for different atoms (white, black, orange, and blue)
- Nose plier for post-processing
Prerequisites
-
Successfully installed Creality K1C 3D printer.
-
Successfully installed Creality Print slicing software in your system.
Molecules Covered
| Molecule | Formula | Embossed Letter |
|---|---|---|
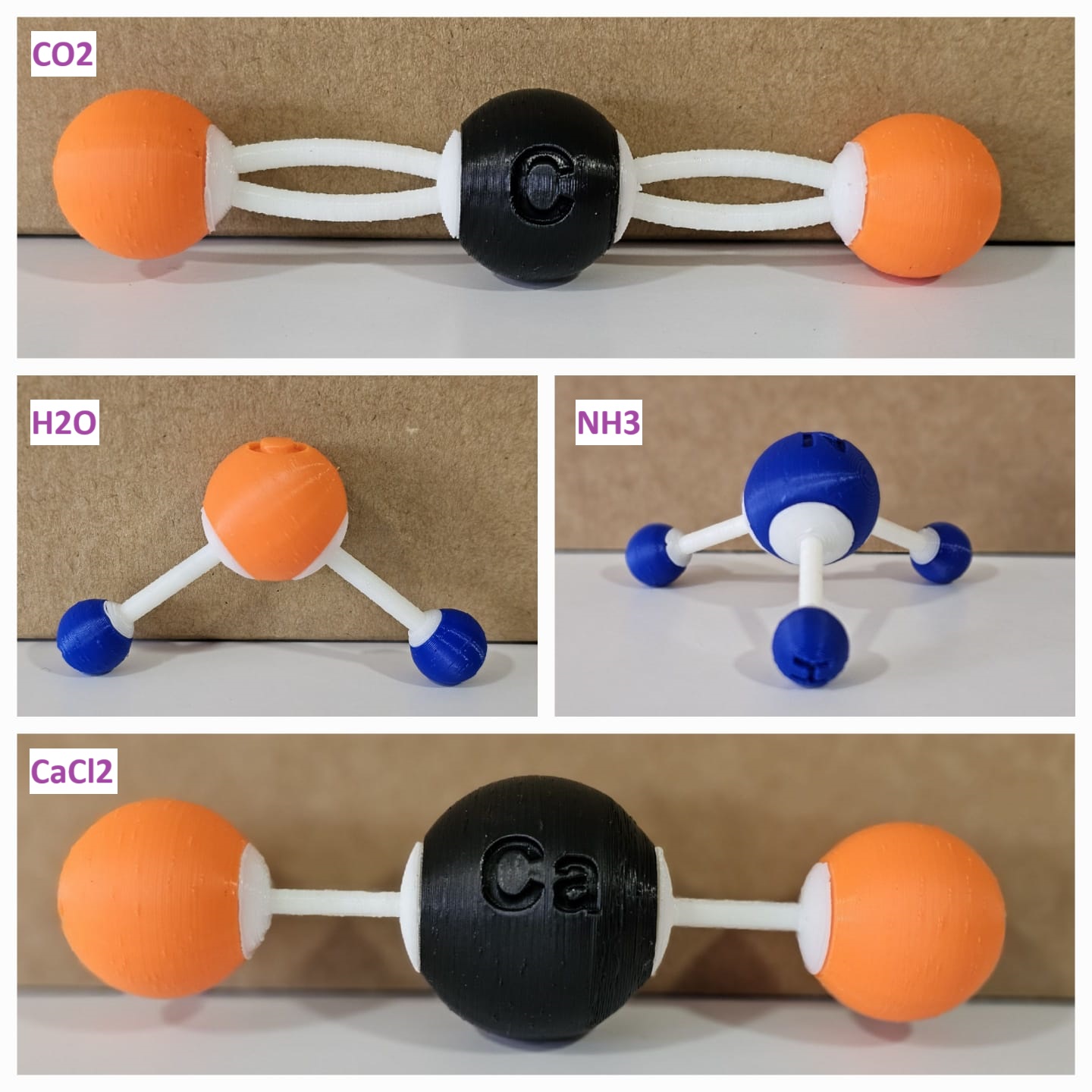
| Water | H2O | H, O |
| Carbon Dioxide | CO2 | C, O |
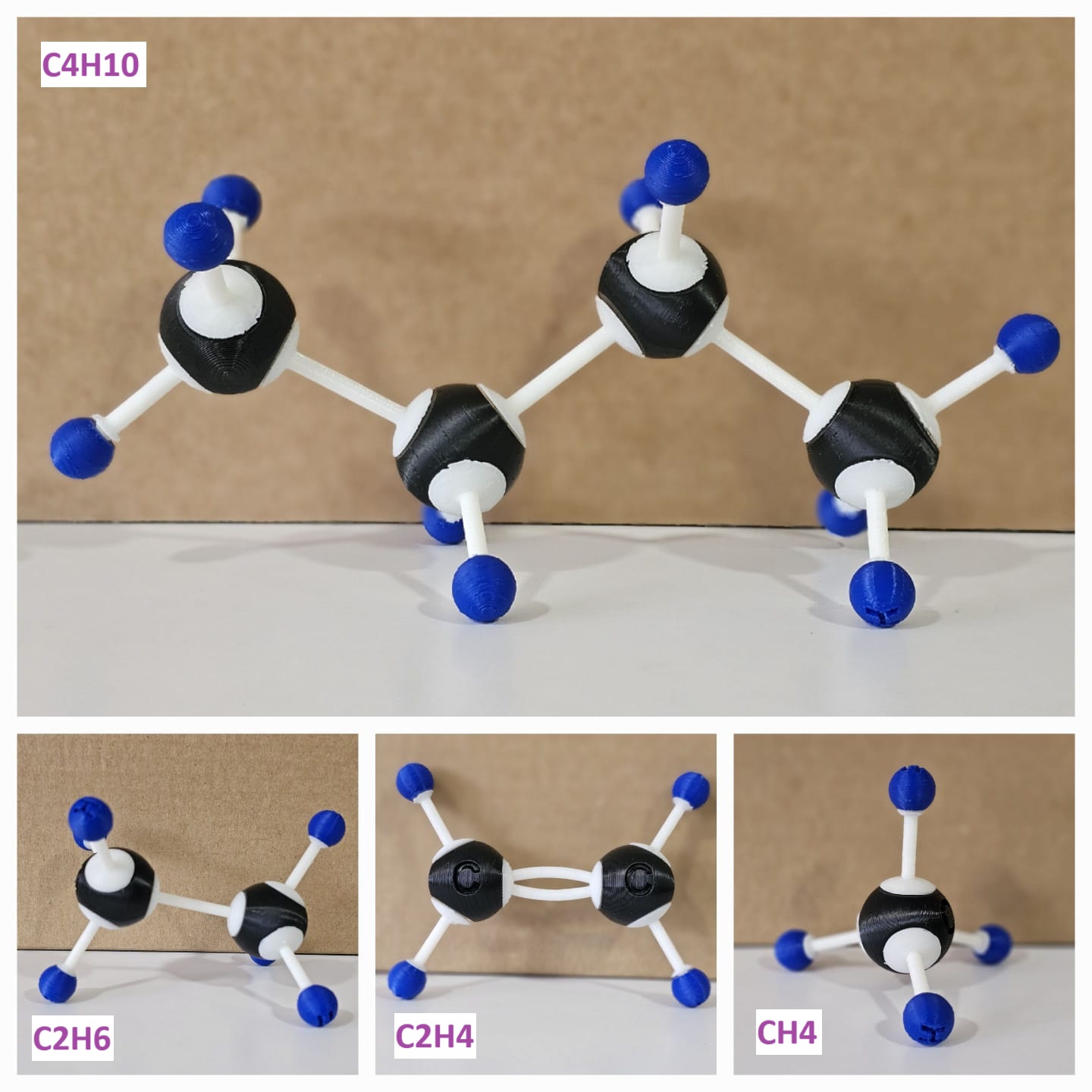
| Methane | CH4 | C, H |
| Ethene | C2H4 | C, H |
| Ethane | C2H6 | C, H |
| Butane | C4H10 | C, H |
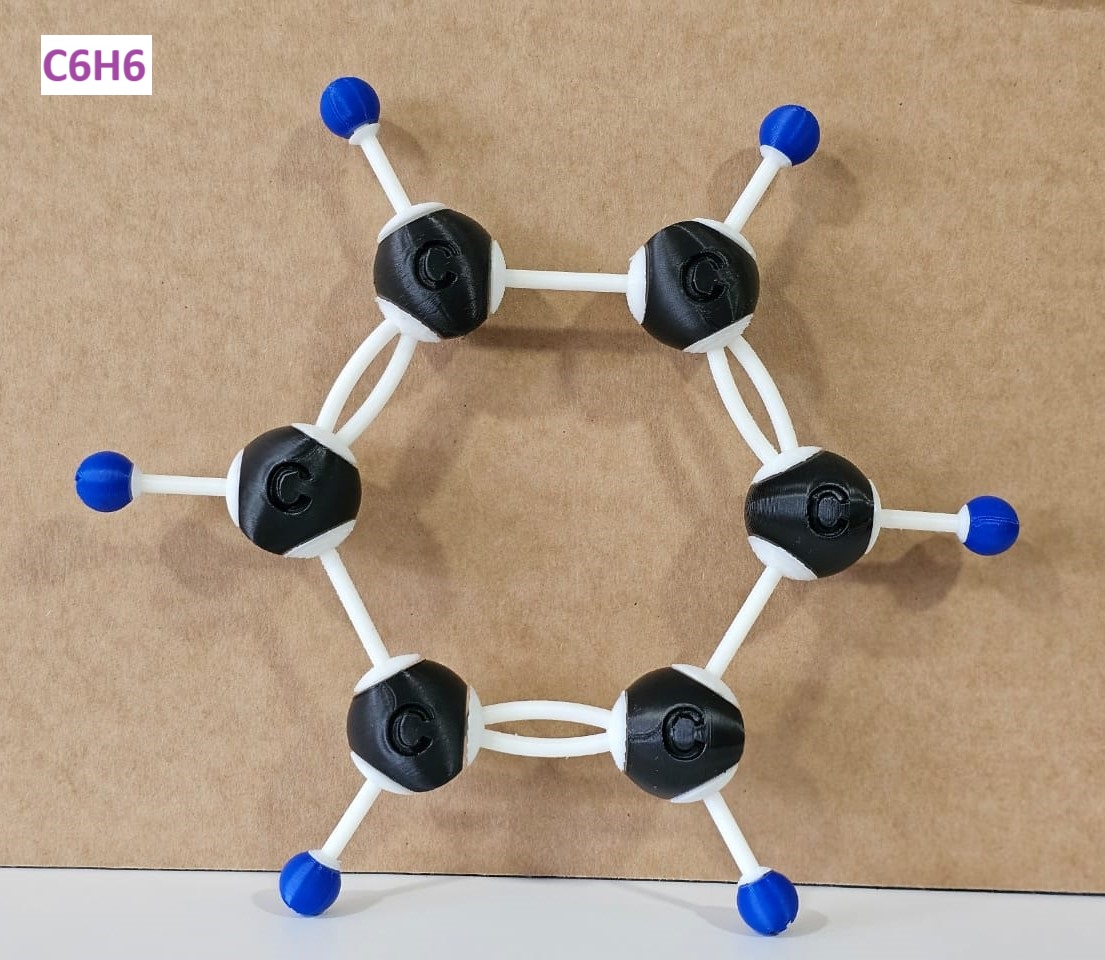
| Benzene | C6H6 | C, H |
| Ammonia | NH3 | N, H |
| Calcium Chloride | CaCl2 | Ca, Cl |
Basic Standards
Available filament colors: White, Blue, Black, and Red
| Atoms and bonds | Symbol | Size (in mm) | Filament Color |
|---|---|---|---|
| Carbon | C | ⌀ 28 mm | Black |
| Hydrogen | H | ⌀ 13 mm | Blue ( or White) |
| Oxygen | O | ⌀ 24 mm | Orange |
| Nitrogen | N | ⌀ 26 mm | Blue |
| Chloride | Cl | ⌀ 30 mm | Orange |
| Calcium | Ca | ⌀ 40 mm | Black |
| Single and double-bond | - , = | White |
Activity 1: 3D-Printed C2H4 Molecule
How to Perform this Activity
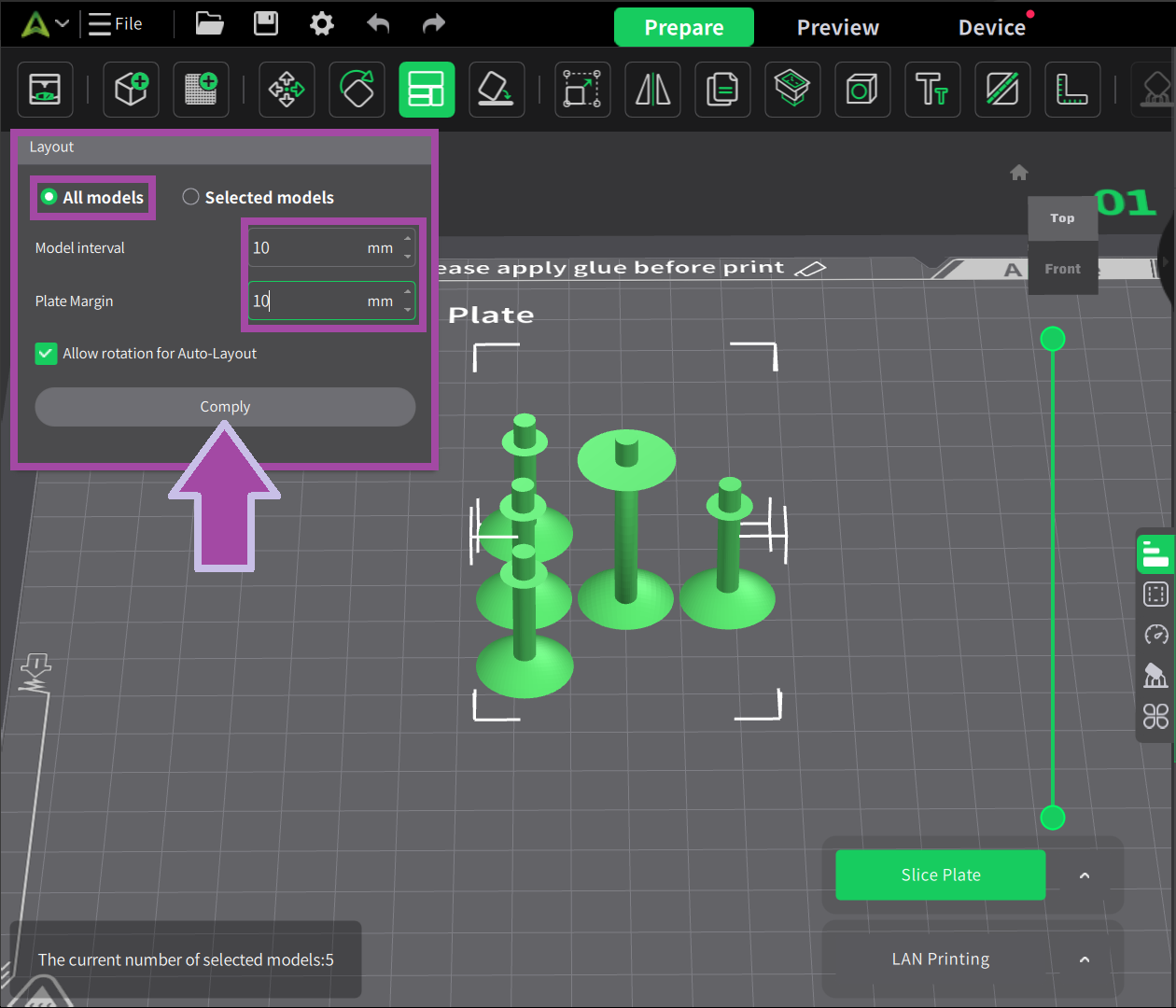
There are three main methods to perform this activity.
- Based on the molecules, print the atoms and bonds.
- Based on the colors, print the atoms and bonds.
- Print all the atoms and bonds in a single color.
It is always preferable to go with either method 1 or method 2 .
Method 1 is preferred when you want to print the molecules chapter-wise, and Method 2 is preferred when you want to print and assemble all the molecules at once.
The detailed documentation to perform this activity is provided below. However, if you find it long and you want to get it done even more quickly, I suggest you follow the videos mentioned below. The video involves the same procedures that are mentioned in this documentation.
Select the molecules that you want to make. For the demonstration purpose, I chose an ethene (C2H4) molecule . Using this molecule, I could demonstrate all the processes involved in this activity, and you could replicate the same process for the rest of the models.
The image below shows the rendered picture of the ethene.

In C2H4, H is in blue color, C is in black color, and bonds are in white color.
3D Printing
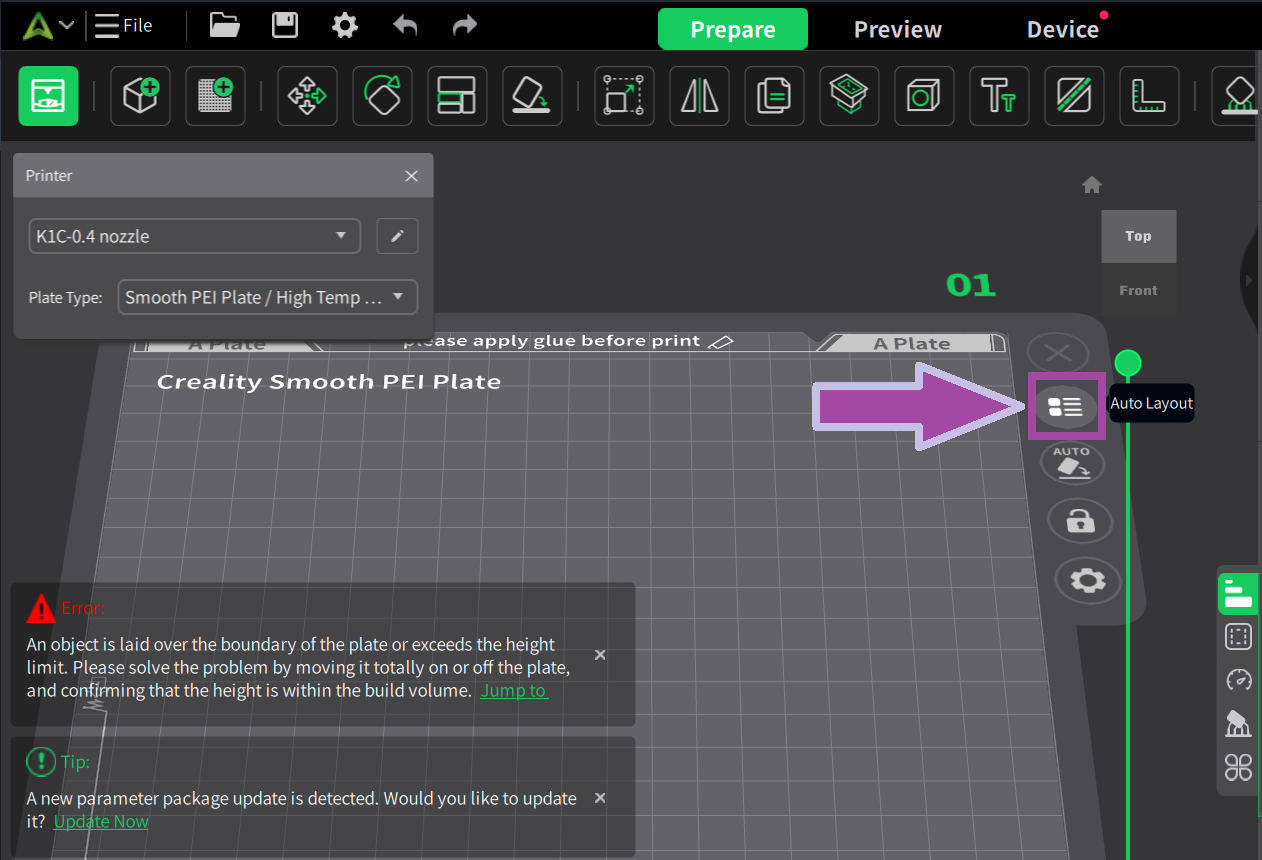
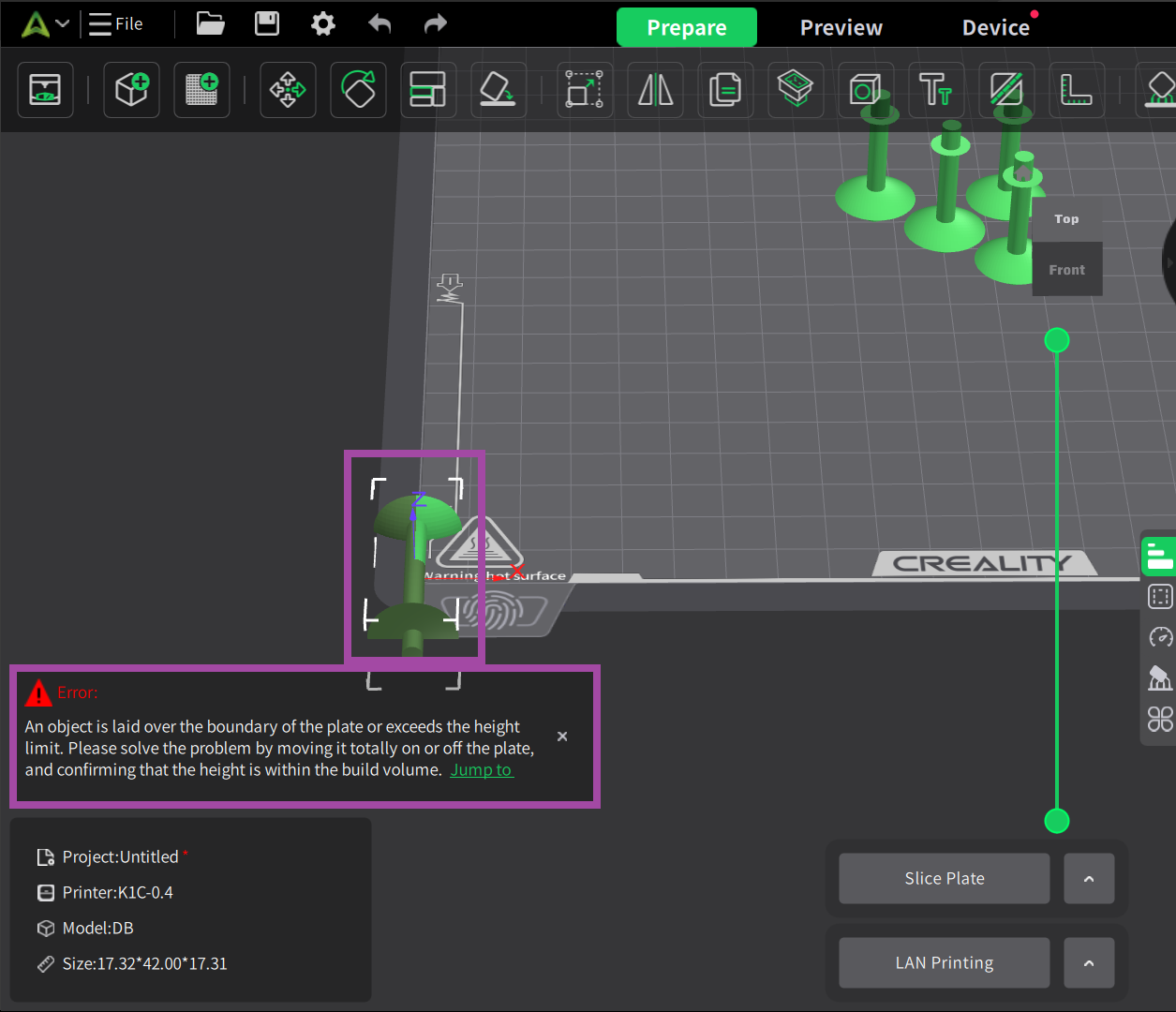
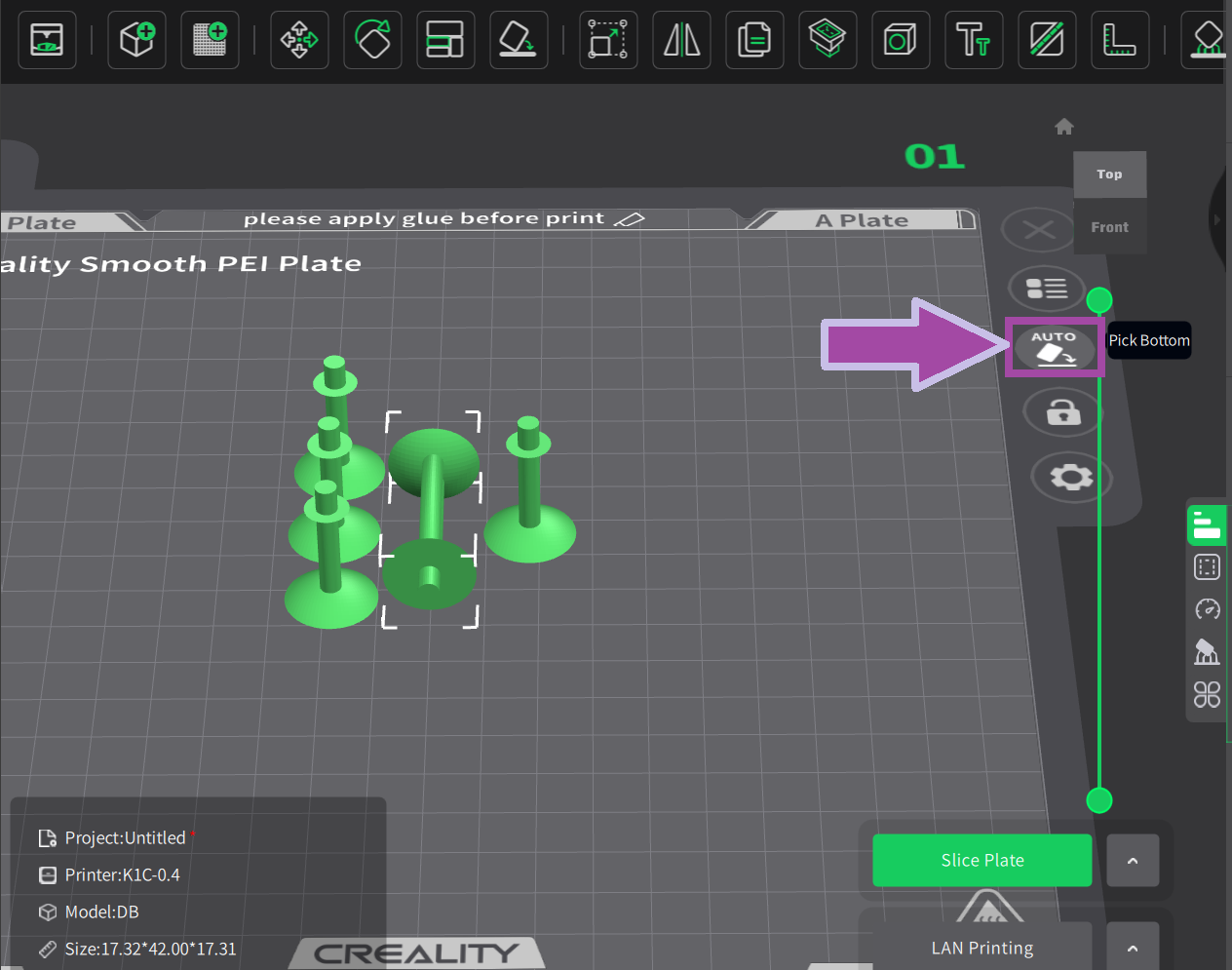
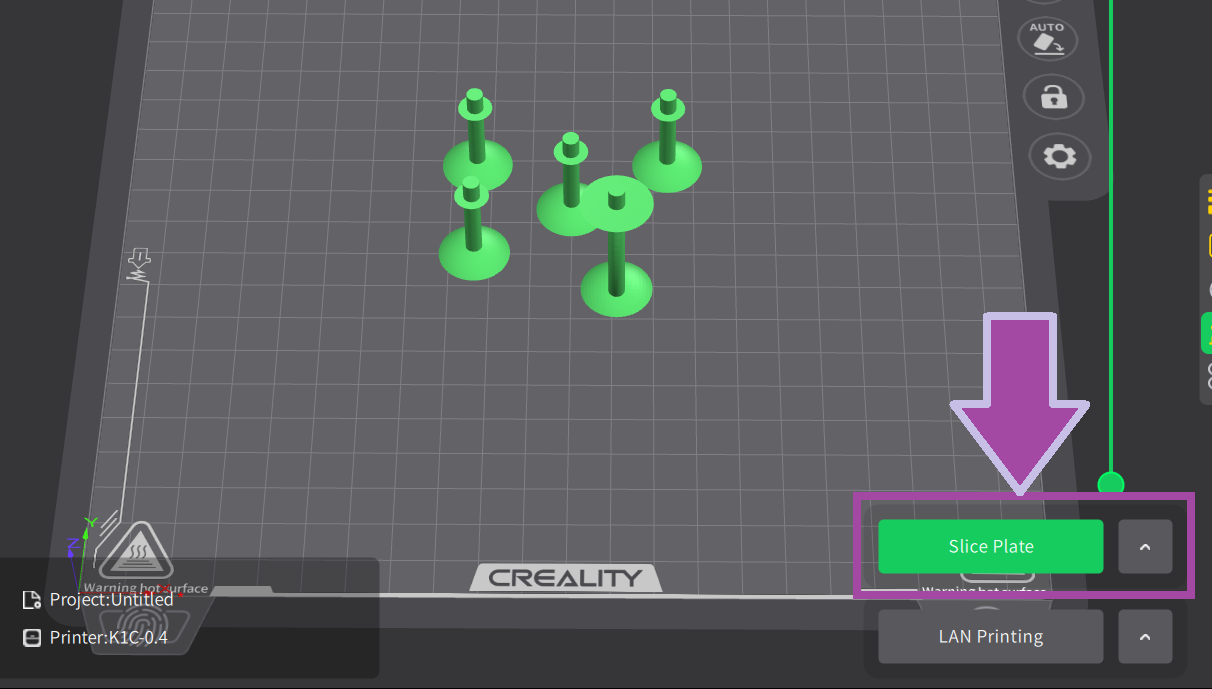
Make sure your 3D printer and Slicer software are ready. If not, click here .
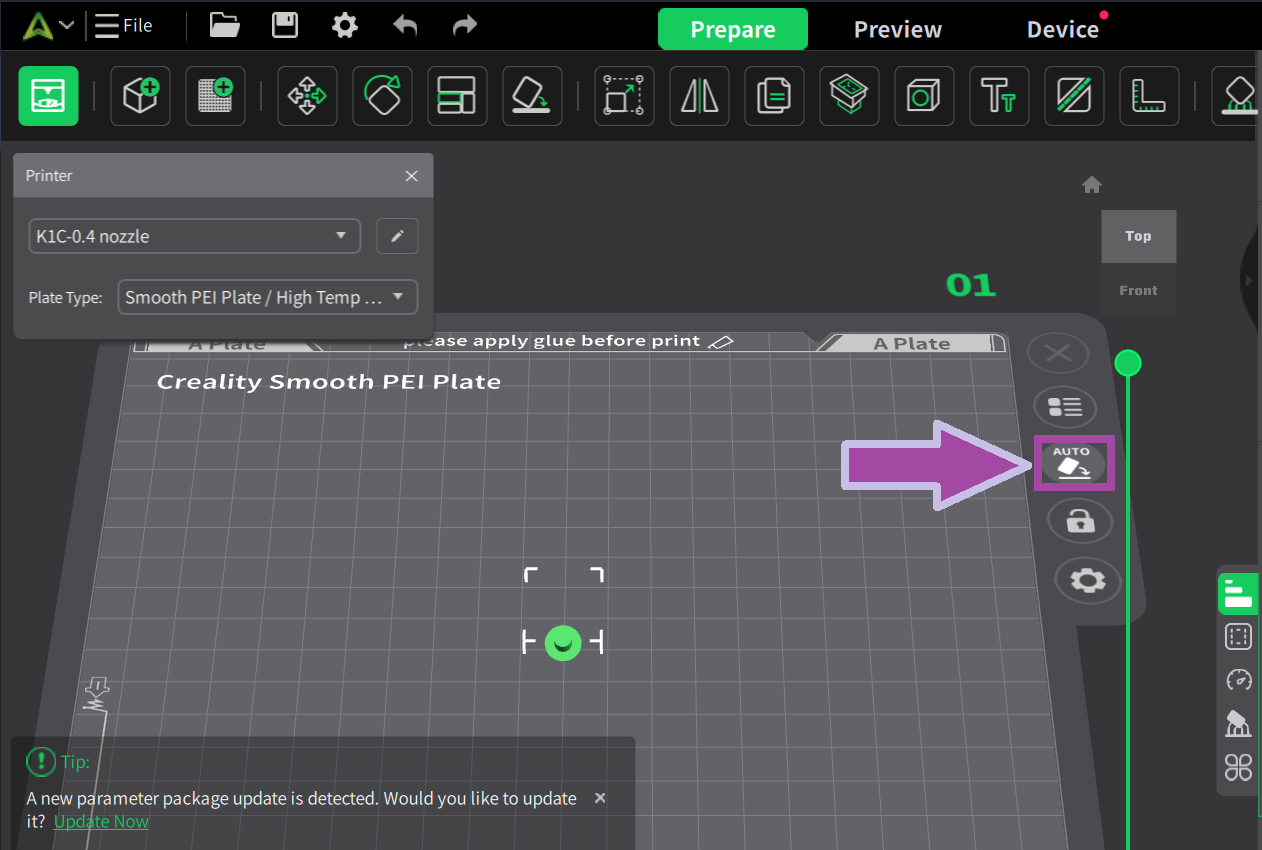
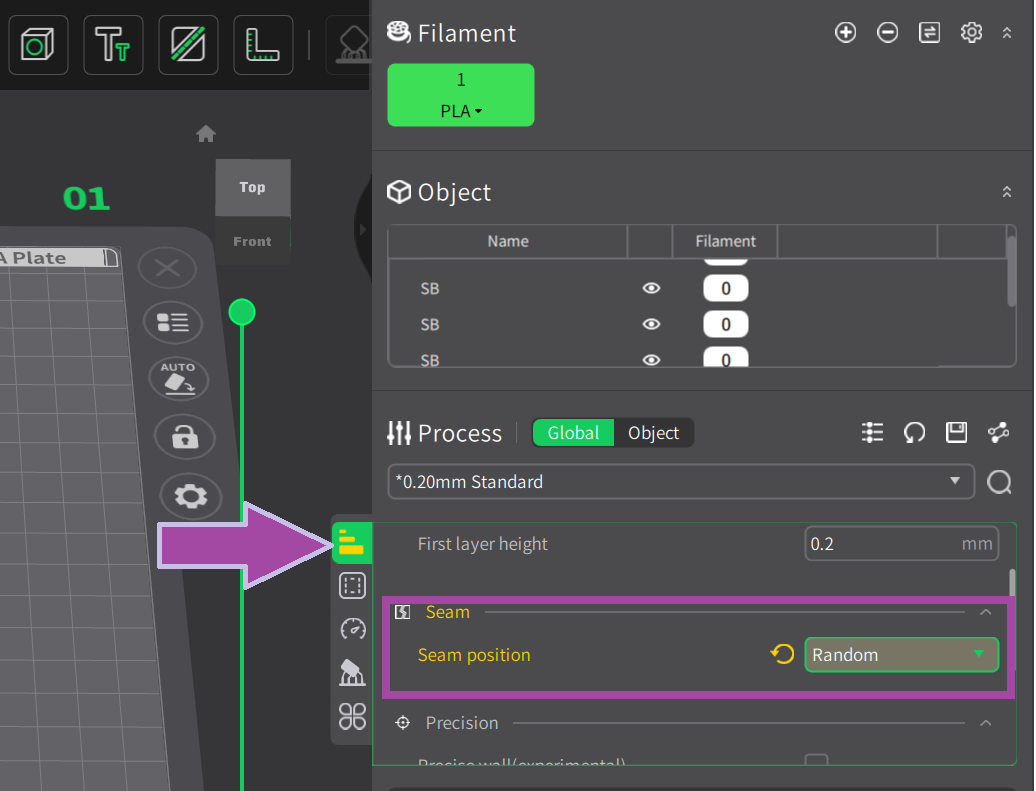
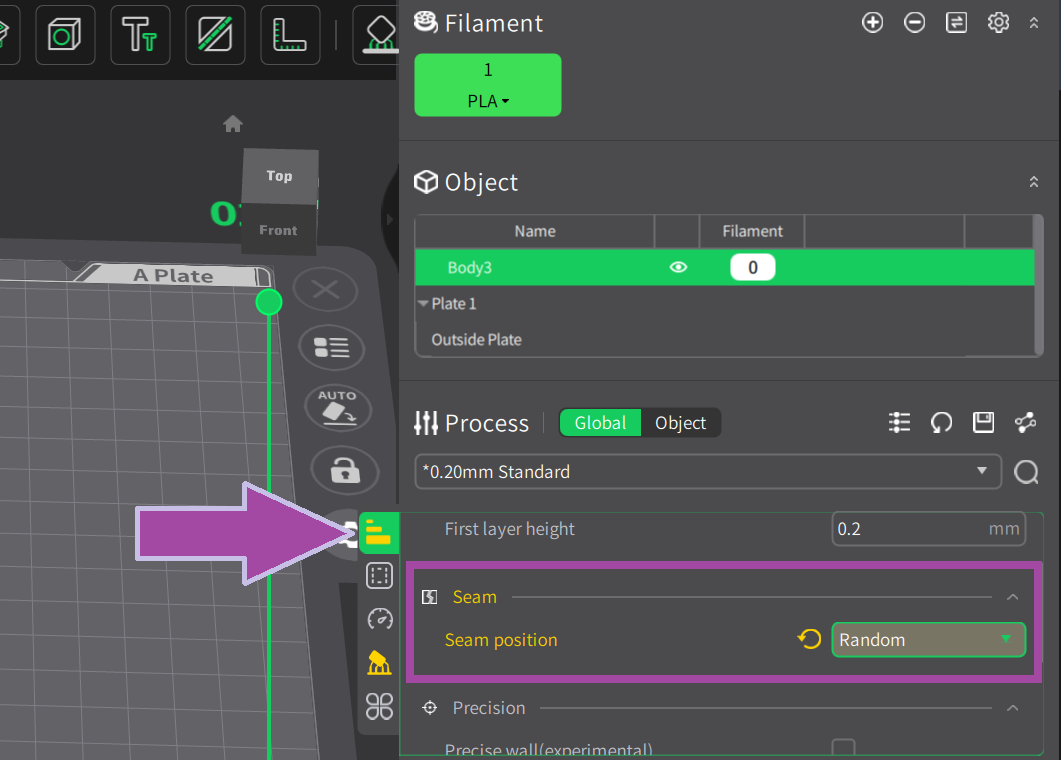
Most likely, the filament color will be white. In the C2H4 molecule, the bonds are white. So, let's first print the C2H4 bonds (single and double bonds).
To learn: How to slice the single and double bonds of the ethene (C2H4)? Follow the link below.
Now let’s print the single and double bonds of ethene.
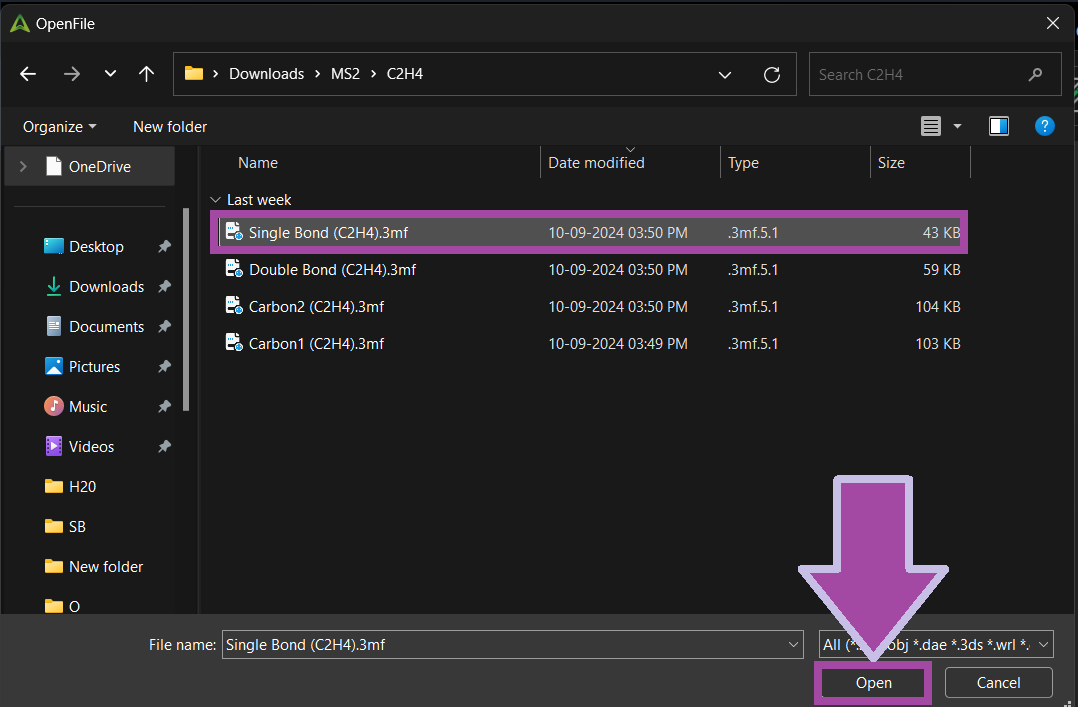
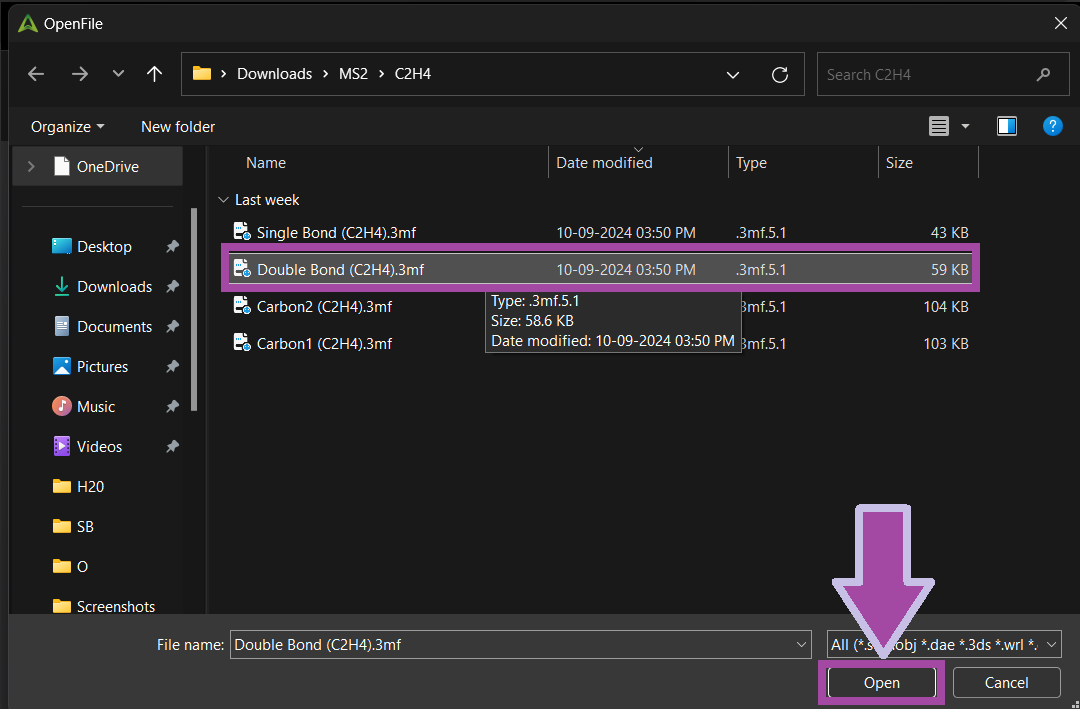
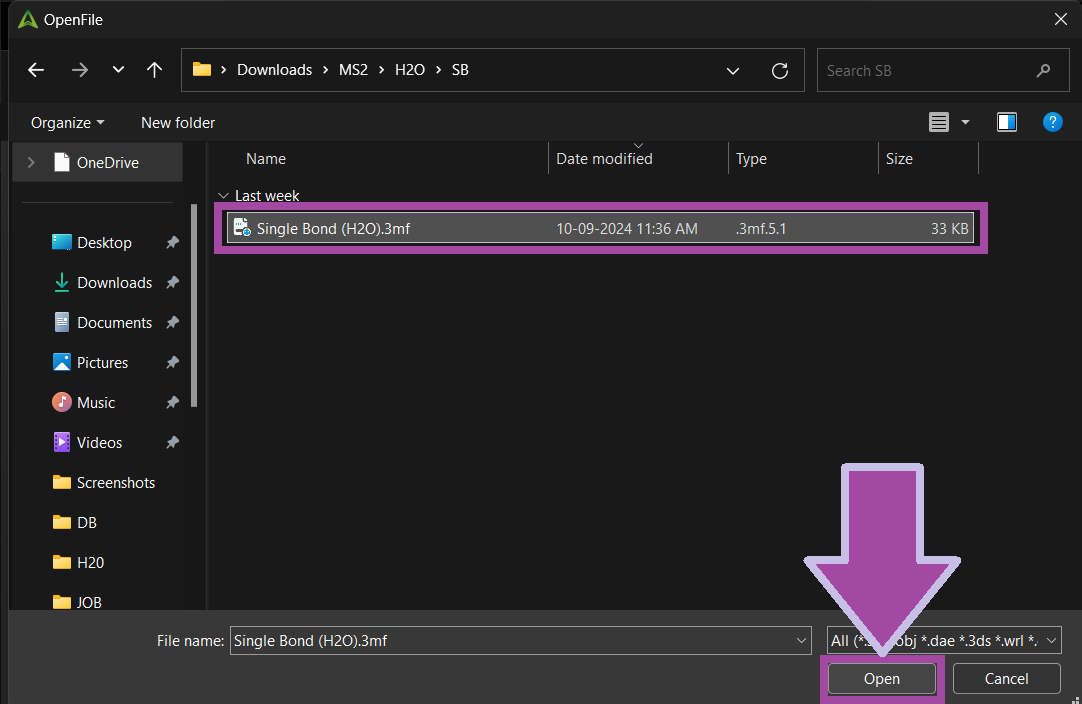
You can download the single bond's 3MF file from the " Resources and Downloads " section below.
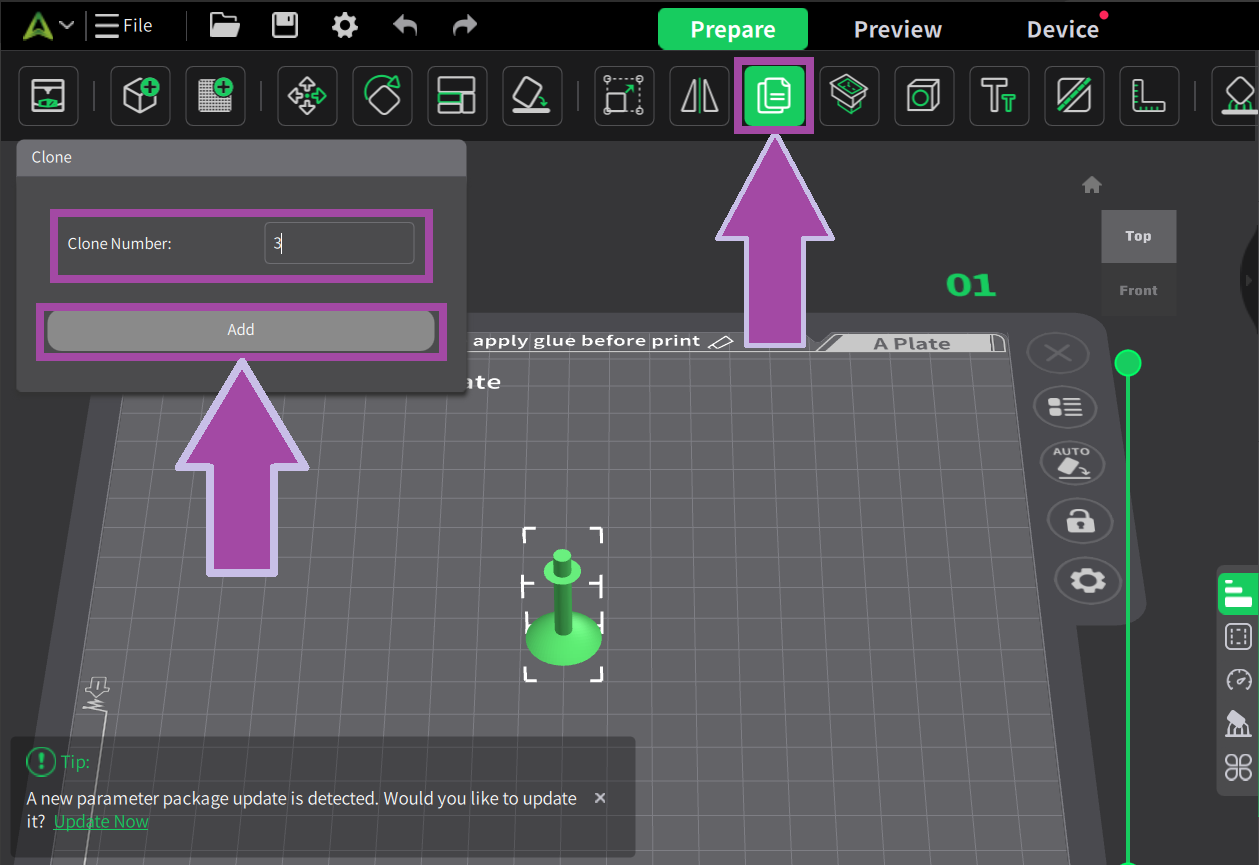
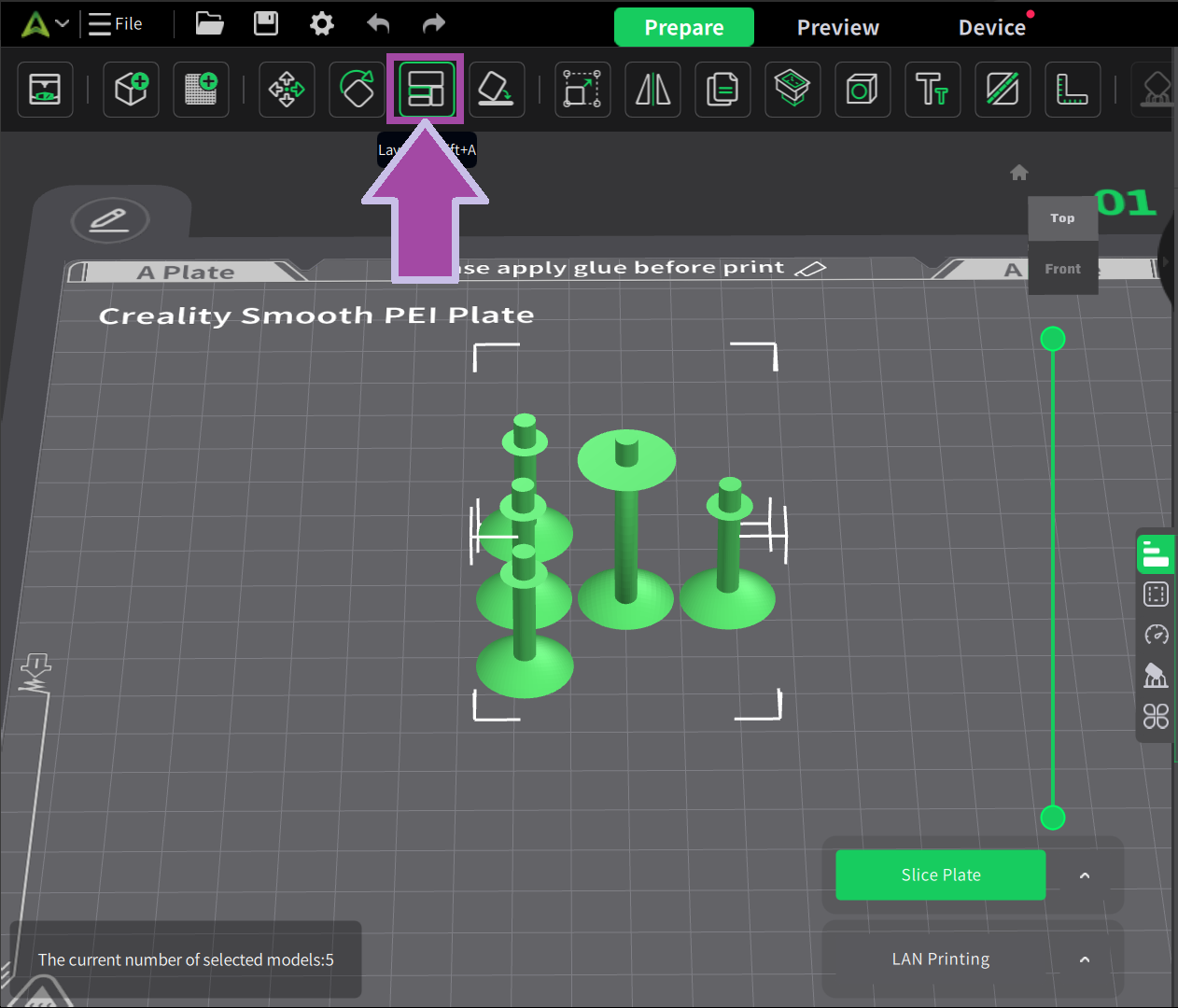
There are four single bonds for C2H4 molecules. So, let’s clone the bond.
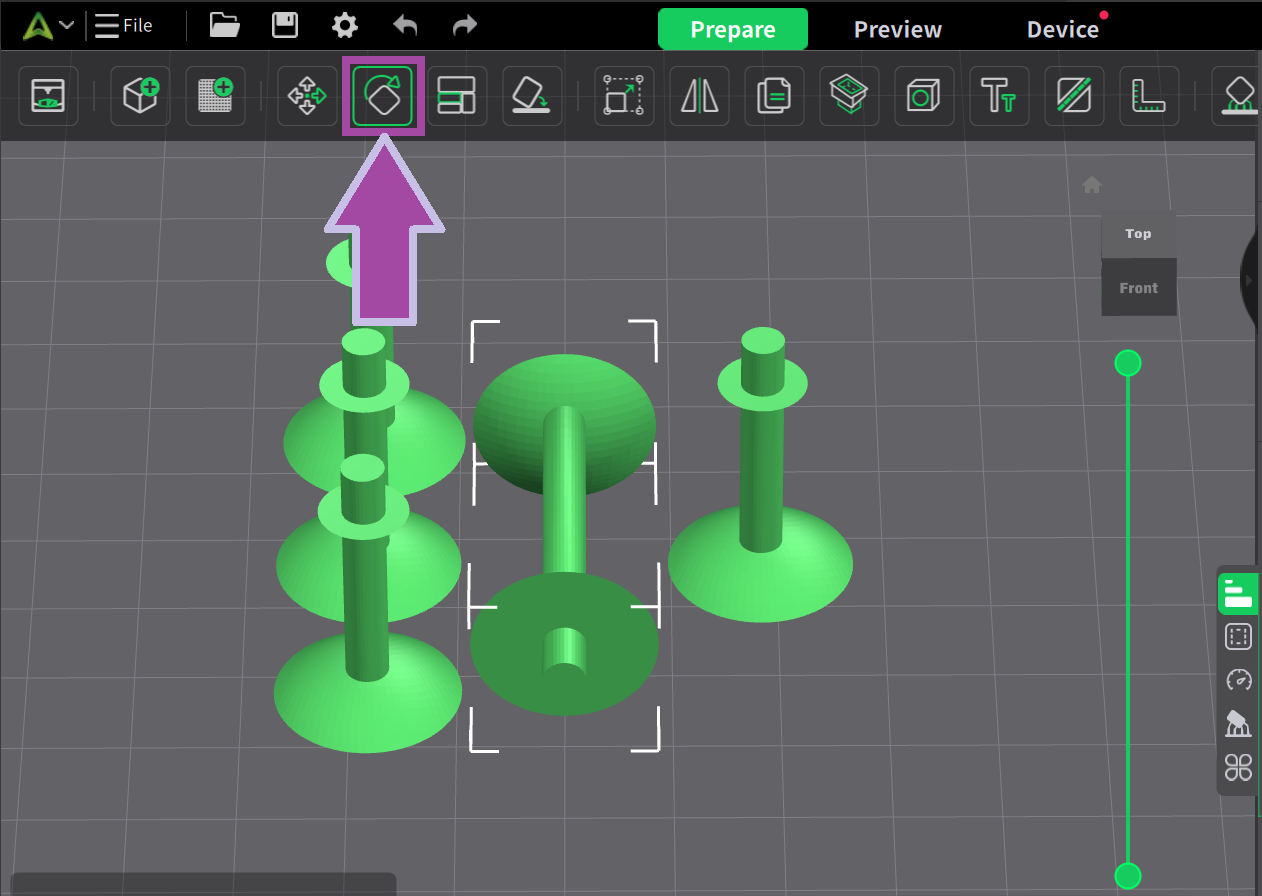
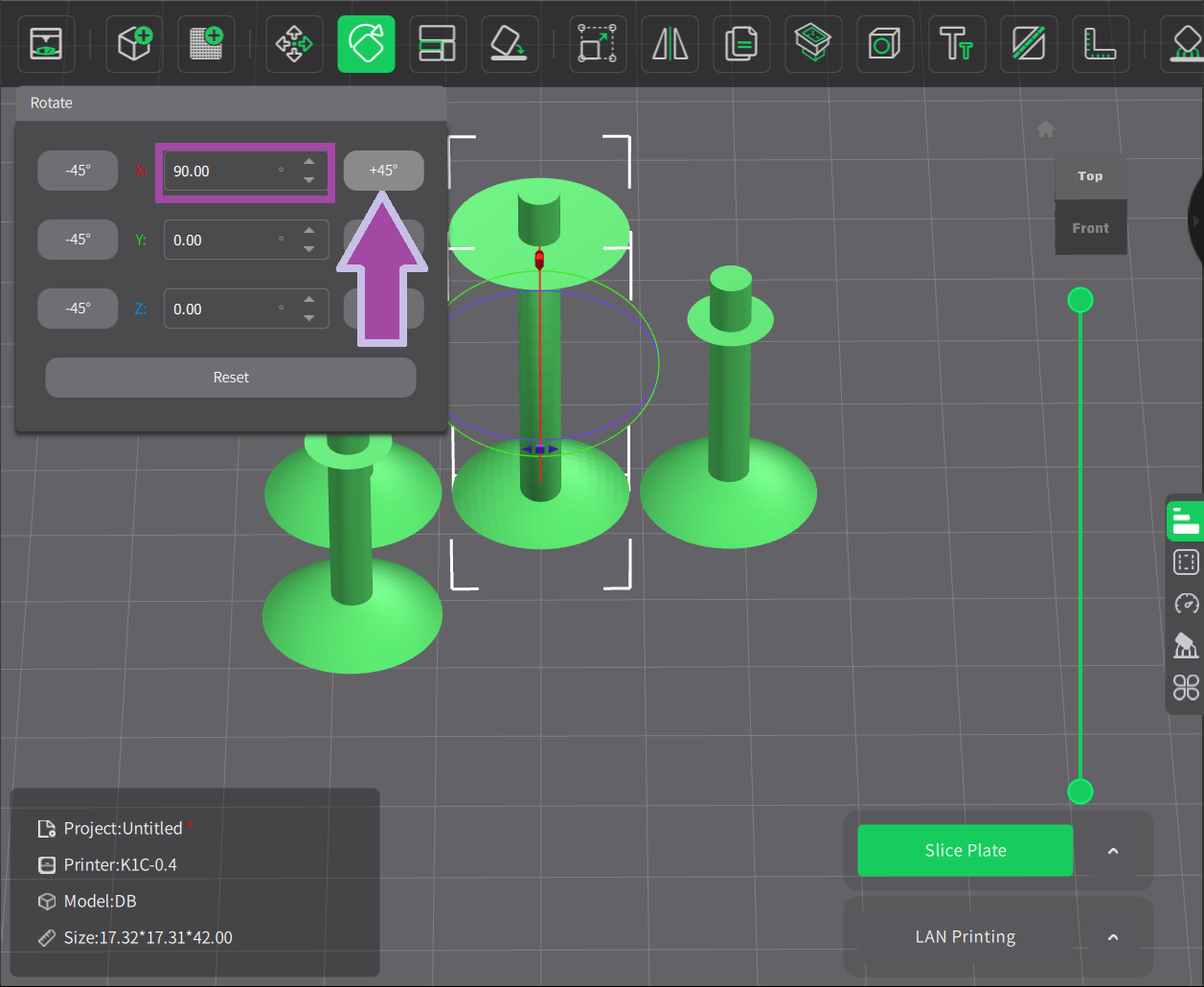
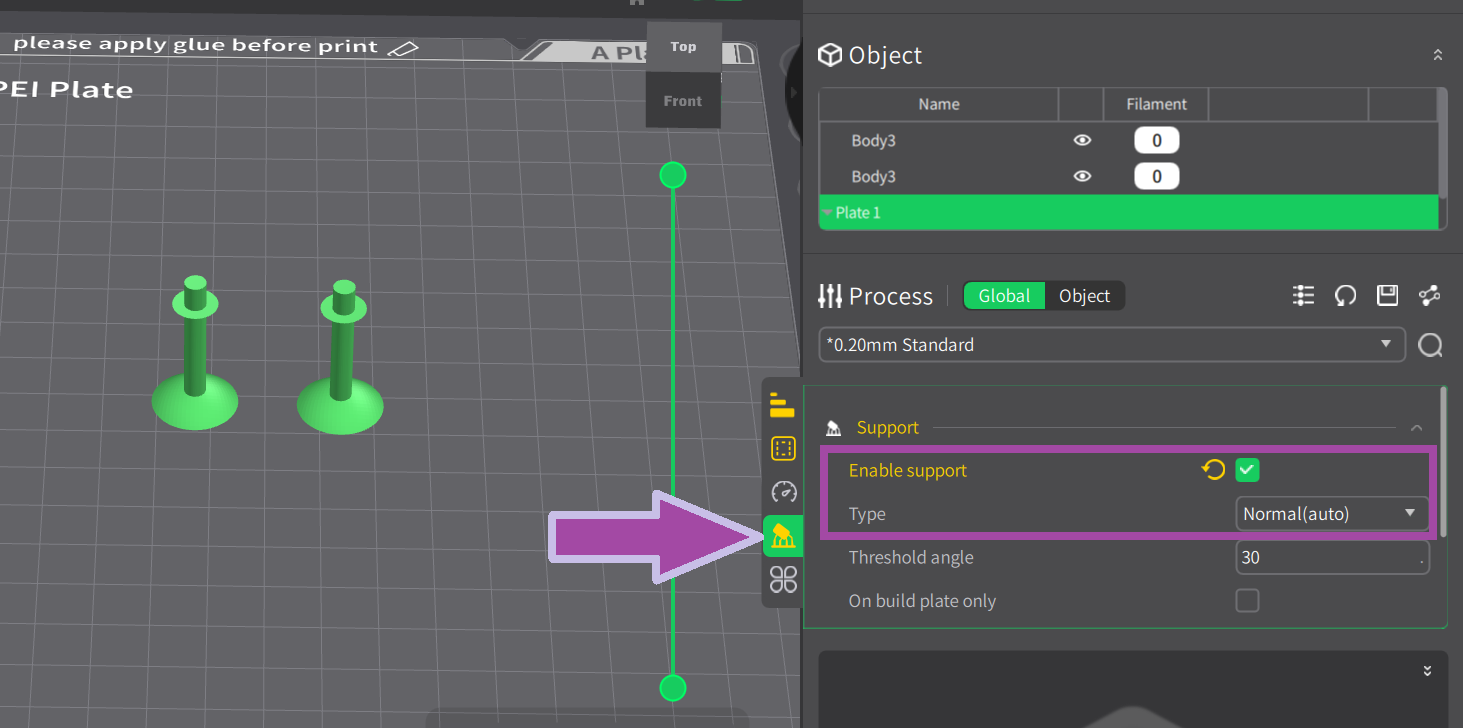
Now it’s time to print the carbon atoms. Let’s change the filament color to black.
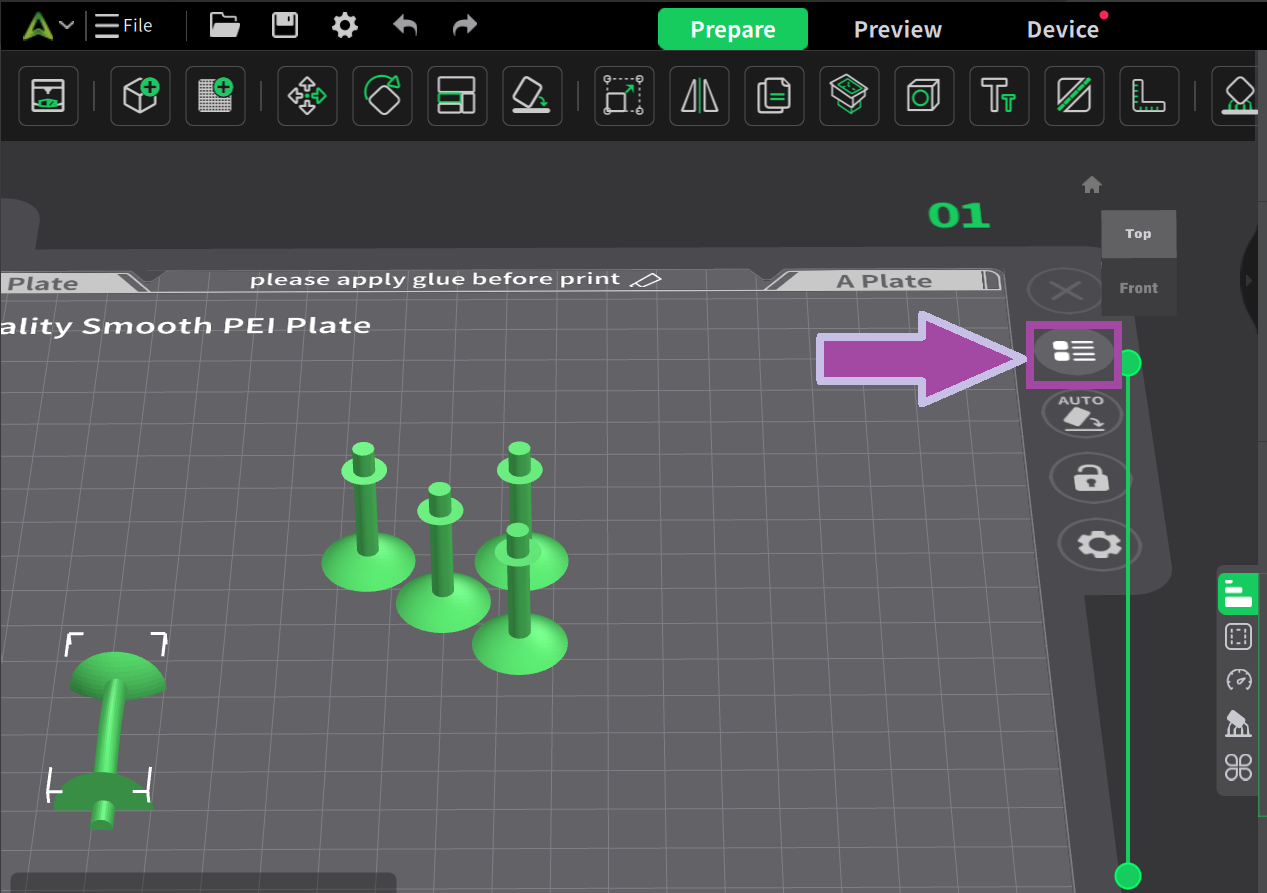
To learn: How to change the filament color? Follow the video mentioned below.
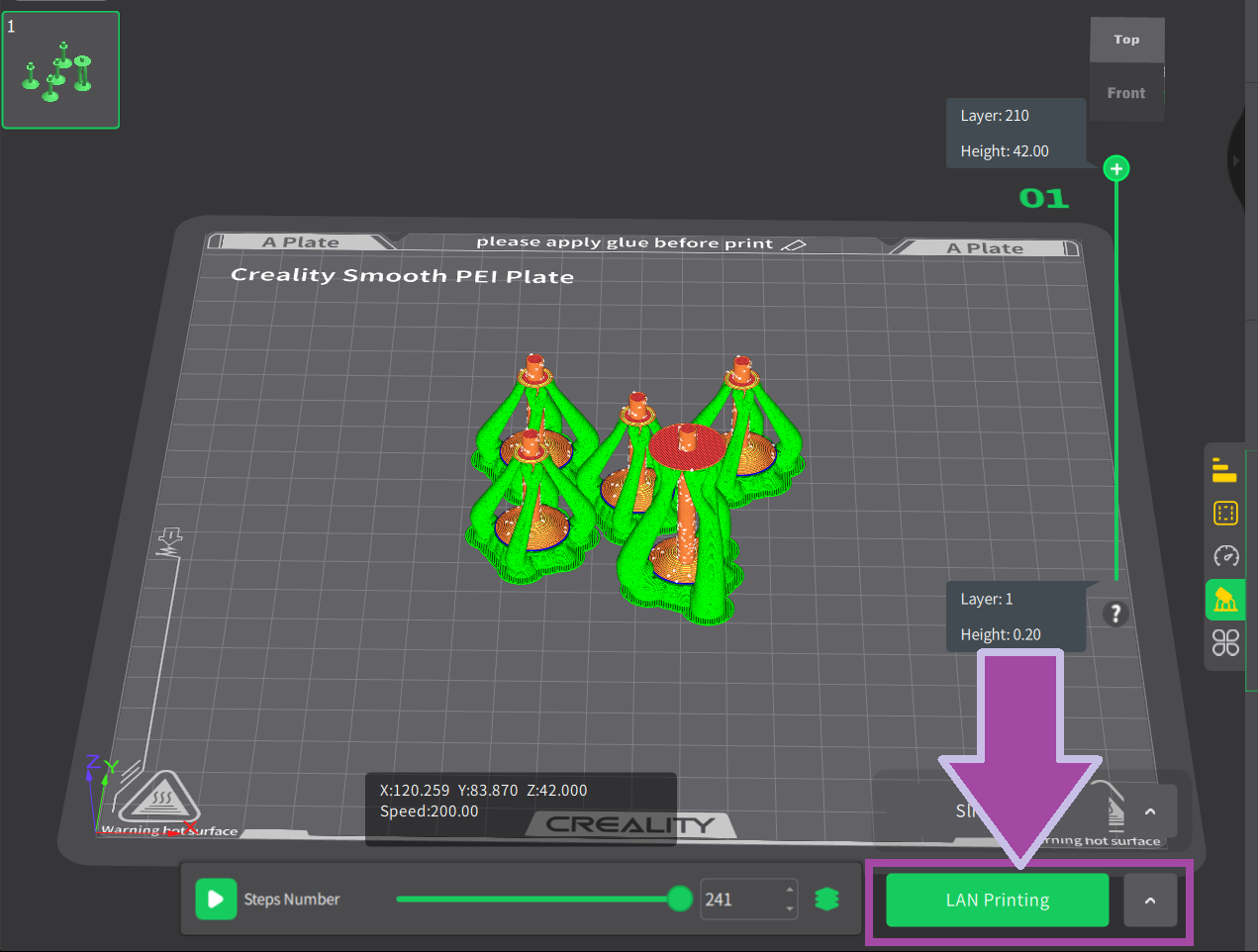
The steps for slicing and printing the carbon and hydrogen atoms are almost the same as above. However, to learn how to slice and print the carbon and hydrogen atoms, follow the video mentioned below.
Download the 3MF file of the carbon atoms from the “ Resources and Downloads ” section.
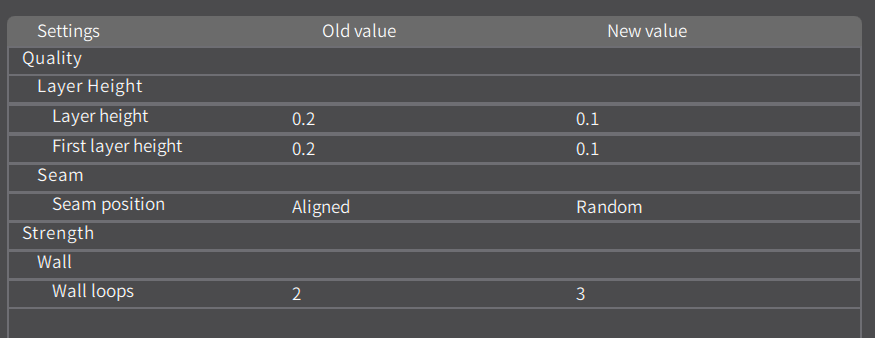
Make sure to set the layer height to 0.1 mm for the carbon and hydrogen atoms.
You can download the hydrogen atoms' 3MF file from the the “ Resources and Downloads ” section.
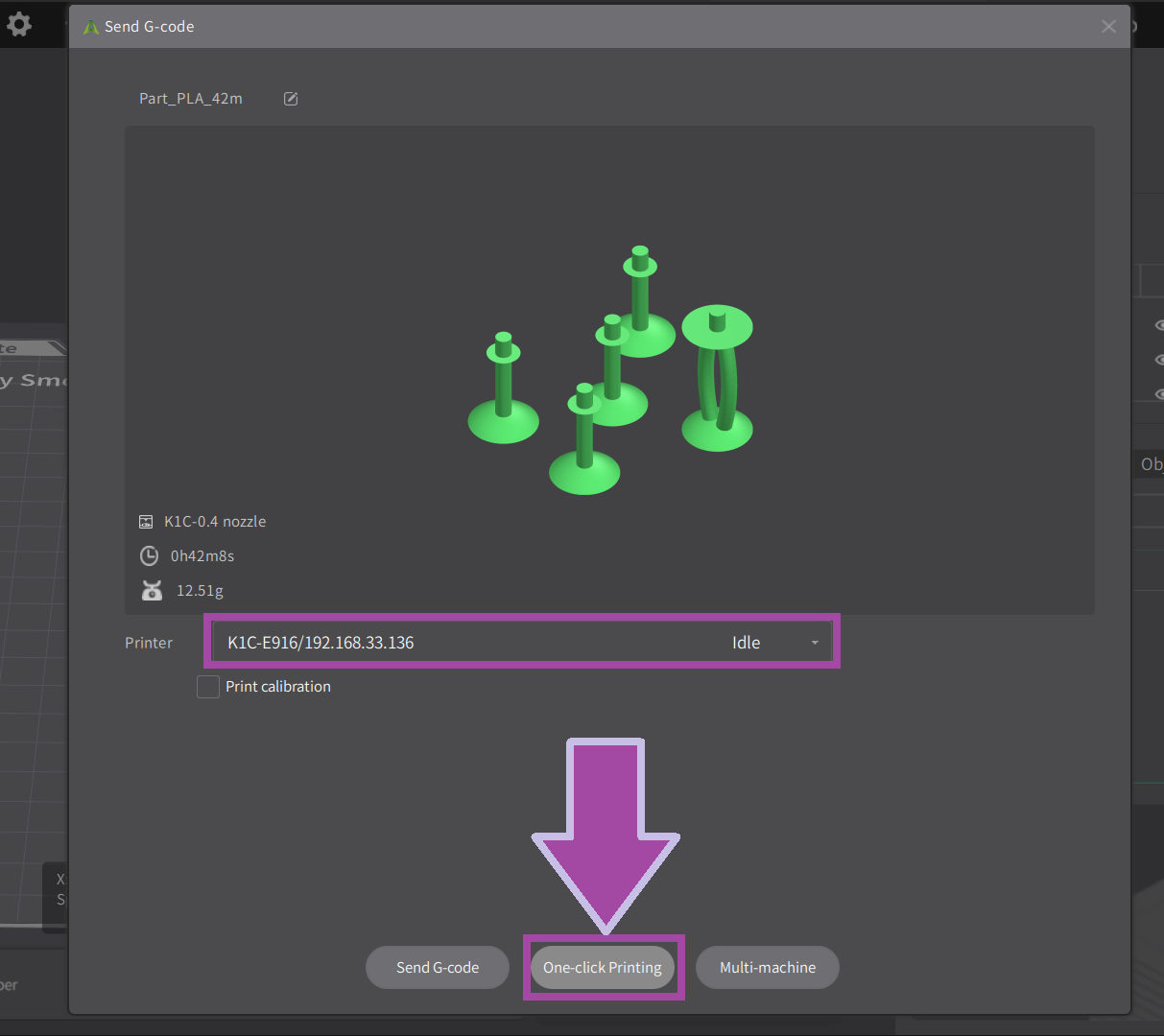
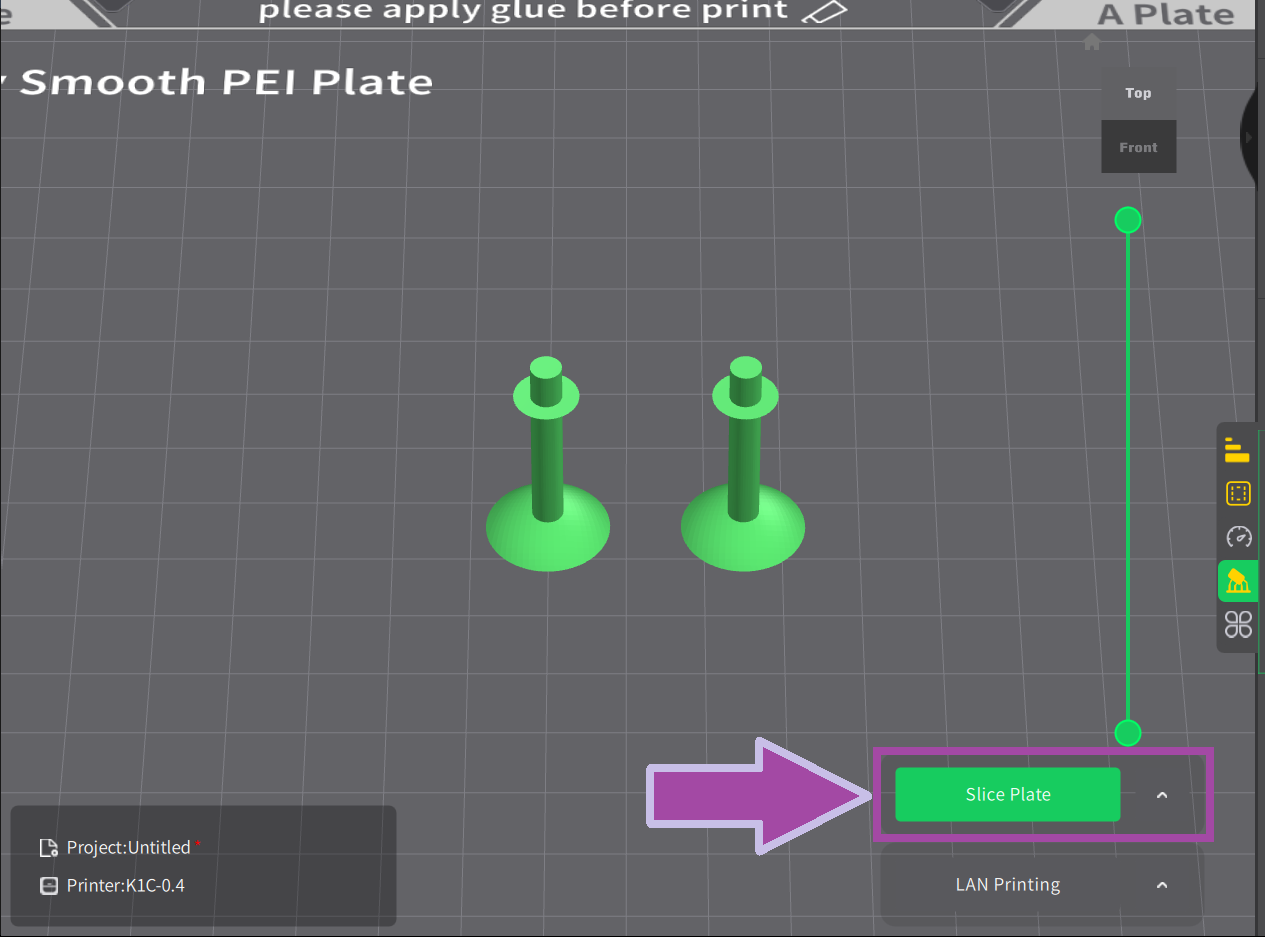
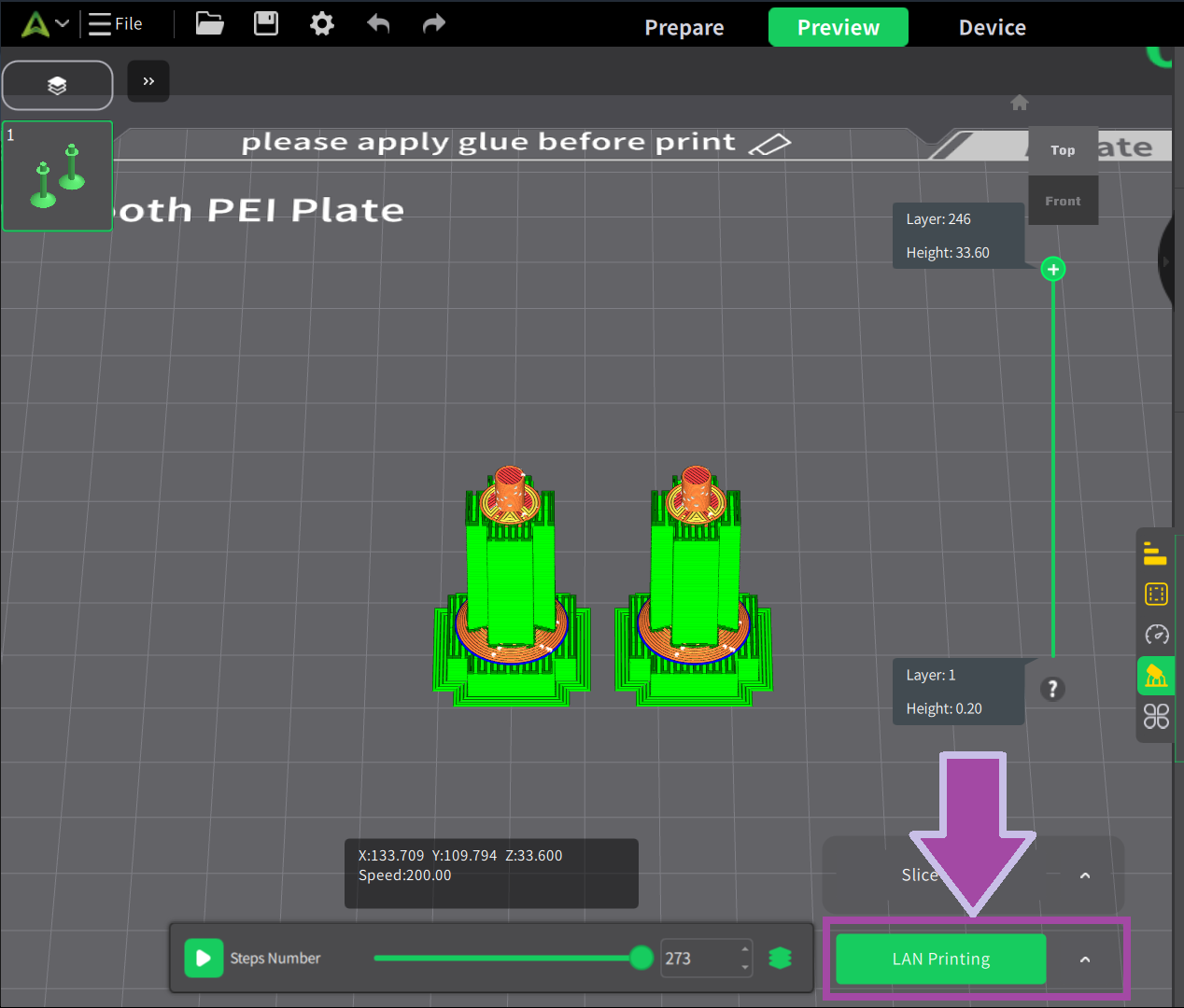
Post-processing
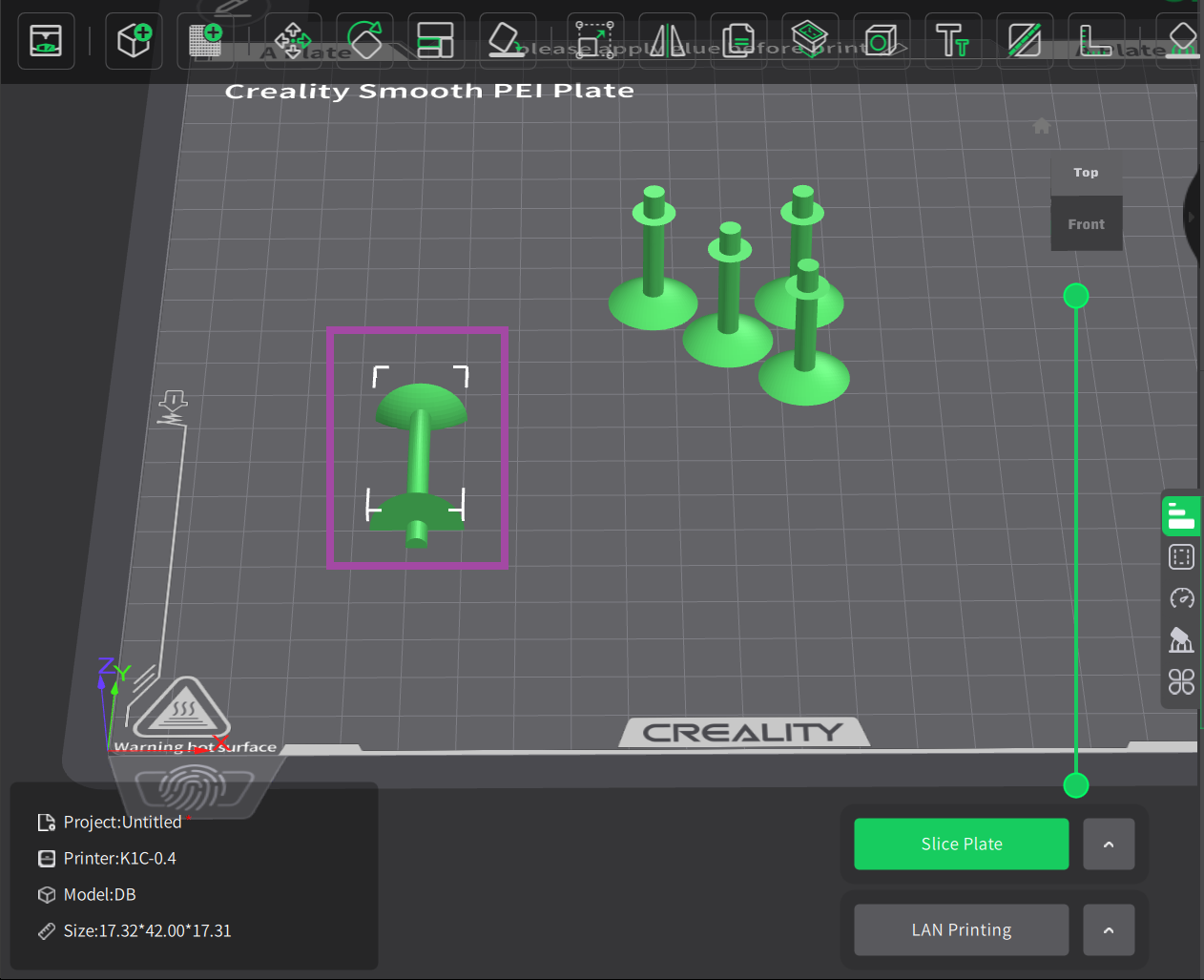
Now it’s time to post-process the printed parts. In the post-process, we remove all the supports, which is not in the actual design.
Assembly
Activity 2: 3D-Printed H2O Molecule
How to Perform this Activity
There are three main methods to perform this activity.
- Based on the molecules, print the atoms and bonds.
- Based on the colors, print the atoms and bonds.
- Print all the atoms and bonds in a single color.
It is always preferable to go with either method 1 or method 2 .
Method 1 is preferred when you want to print the molecules chapter-wise, and Method 2 is preferred when you want to print and assemble all the molecules at once.
3D Printing
Make sure your 3D printer and Slicer software are ready. If not, click here .
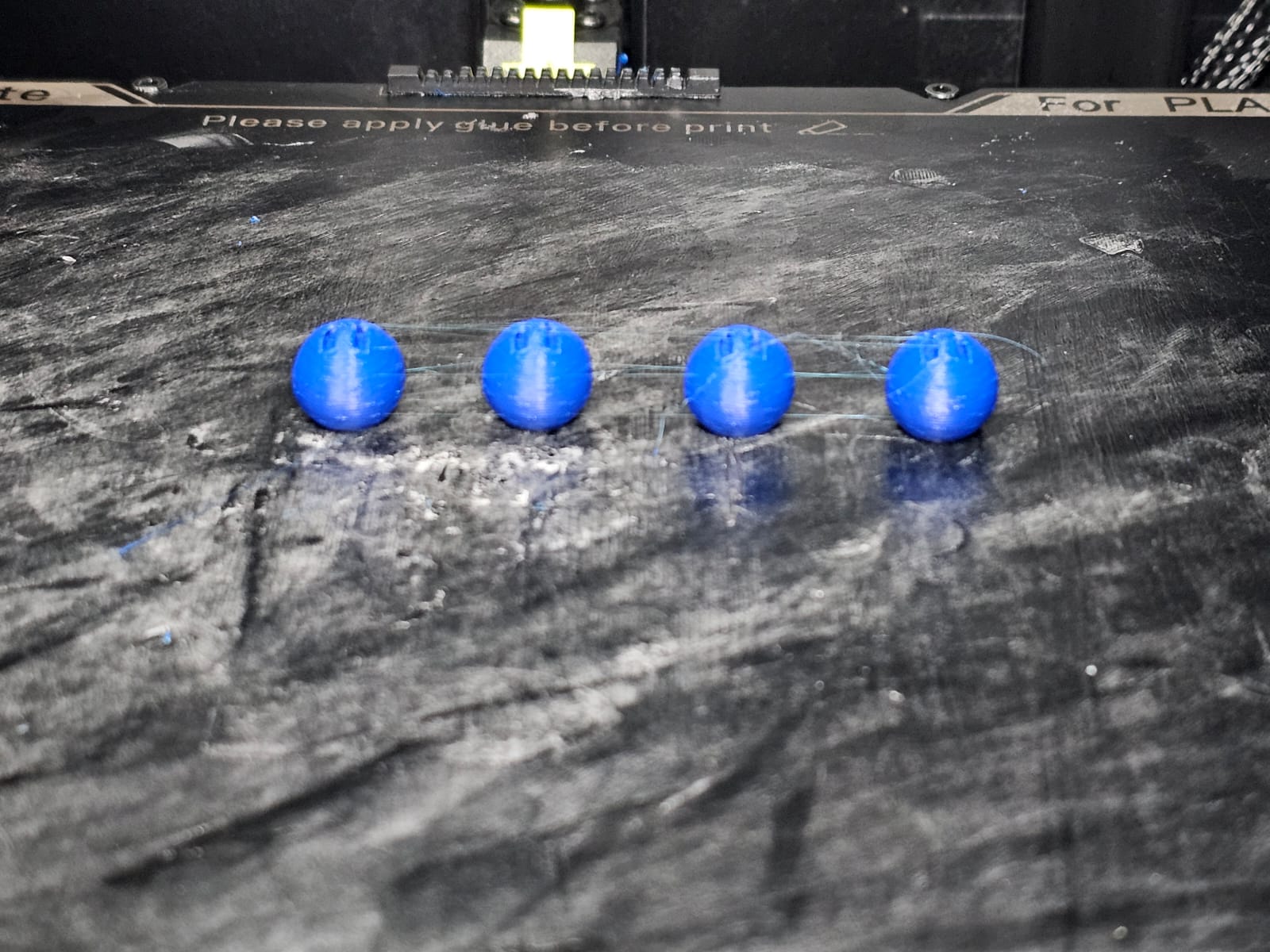
Most likely, the filament color will be white. In the H2O molecule, the bonds are white. So, let's first print the H2O bonds.
 https://youtu.be/F8TzLyNc4OE
https://youtu.be/F8TzLyNc4OE

The single bond 3MF file can be downloaded from the 3MF file section mentioned below.
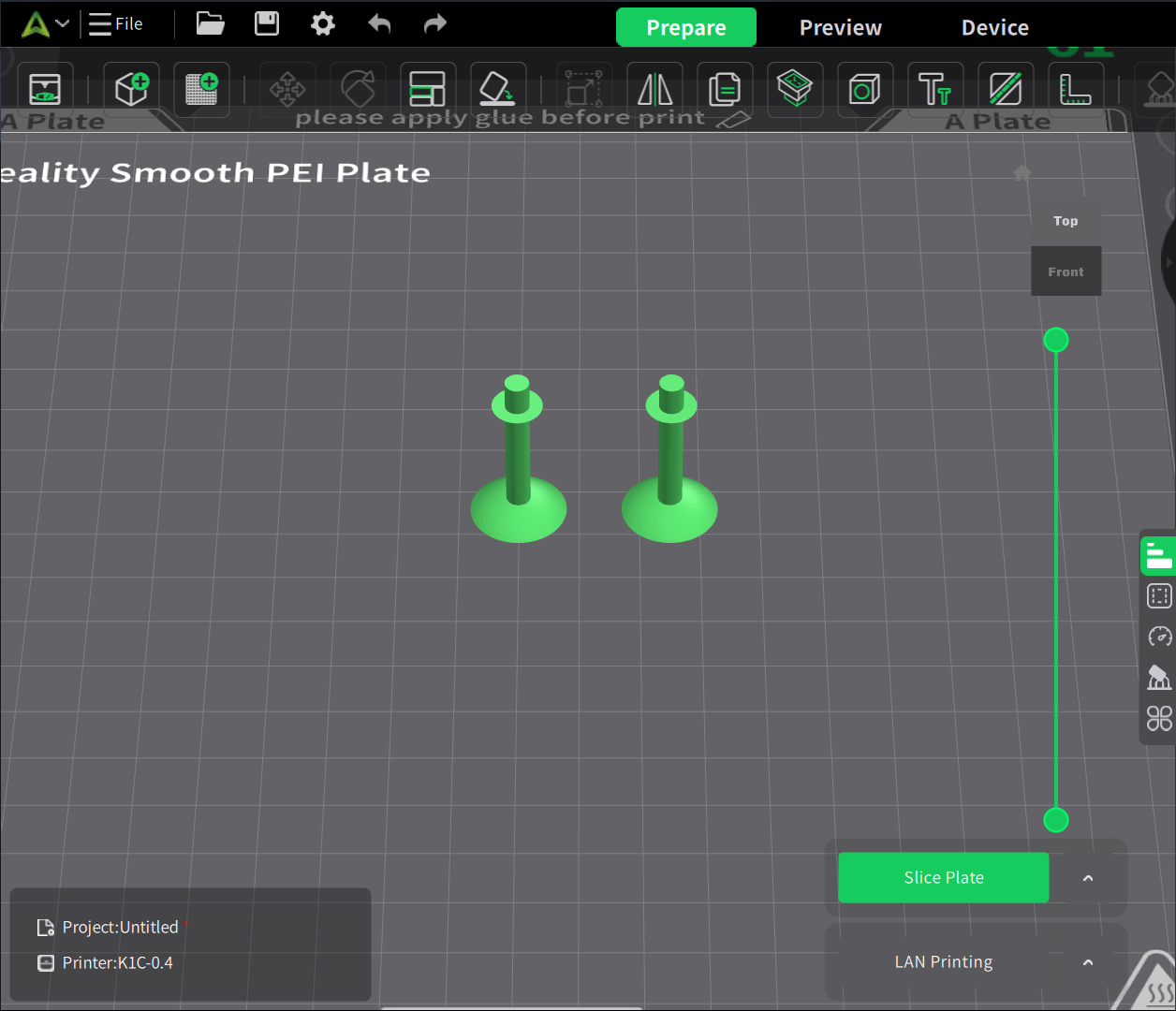
There are two single bonds for H2O molecules. So, let’s clone the bond.
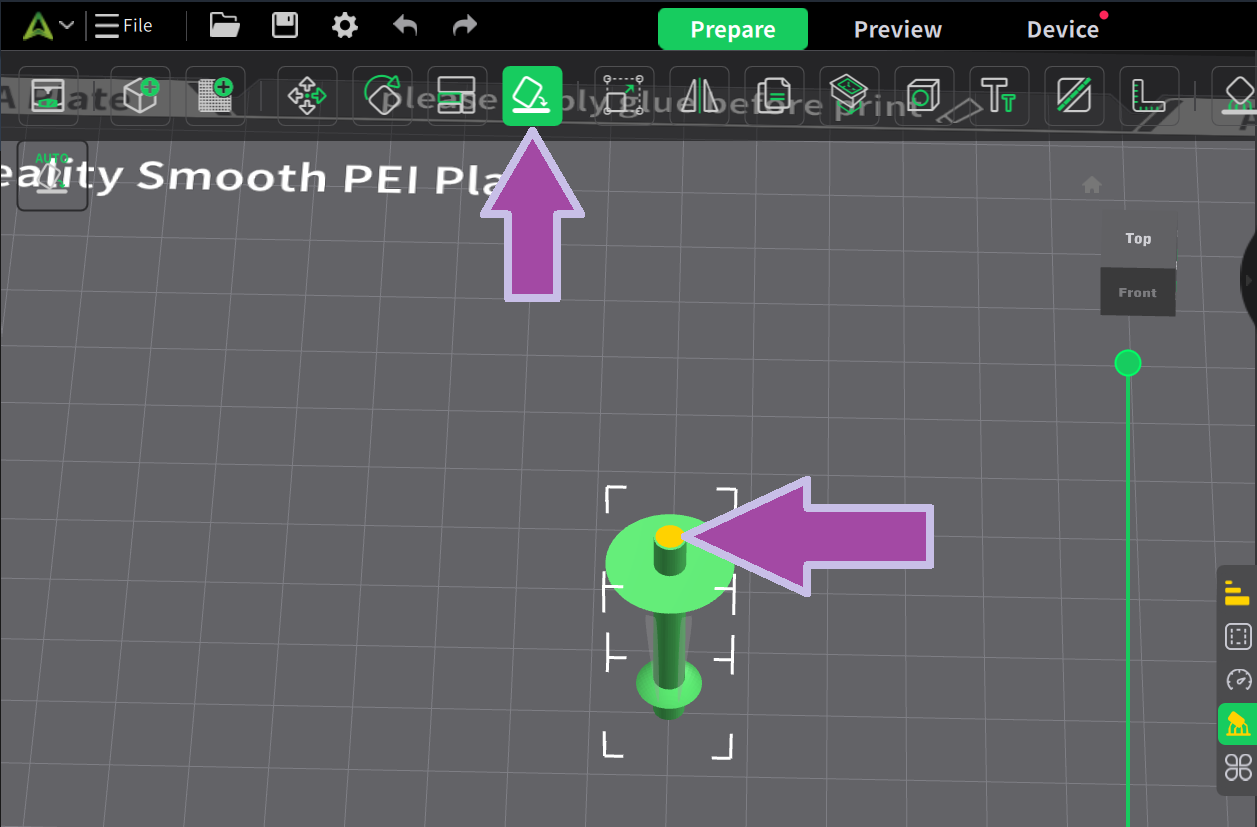
Now it’s time to print the oxygen atom. Let’s change the filament color.
To learn how to change the filament color, click here.
Download the 3MF file of the oxygen atom from the 3MF file section.
Make sure to set the layer height to 0.1 mm.
 https://youtu.be/PqZSahGUVJI
https://youtu.be/PqZSahGUVJI

You can download the Hydrogen 3MF file from the section on 3MF files listed below.
Post-processing
Now it’s time to post-process the printed parts. In the post-process, we remove all the supports, which is not in the actual design.
Assembly
Molecular Structure Checklist
-
H2O (Water)
-
CO2 (Carbon Dioxide)
-
CH4 (Methane)
-
C2H6 (Ethane)
-
C2H4 (Ethene)
-
C4H10 (Butane)
-
C6H6 (Benzene)
-
NH3 (Ammonia)
-
CaCl2 (Calcium Chloride)
3D-Printed Molecular Structures
Resources and Downloads
 https://drive.google.com/drive/folders/11-aMumVKsjtSXye6cR739tBQWoaKiwsQ?usp=sharing
https://drive.google.com/drive/folders/11-aMumVKsjtSXye6cR739tBQWoaKiwsQ?usp=sharing
| METHOD 1 | METHOD 2 |
|---|---|
| 📁 H2O (Water) | 📁 Carbon (C6H6) |
| 📁 CO2 (Carbon Dioxide) | 📁 Carbon (C2H6) |
| 📁 CH4 (Methane) | 📁 Carbon (C2H4) |
| 📁 C2H6 (Ethane) | 📁 Carbon (CH4) |
| 📁 C2H4 (Ethene) | 📁 Carbon (CO2) |
| 📁 C4H10 (Butane) | 📁 Oxygen (H2O) |
| 📁 C6H6 (Benzene) | 📁 Oxygen (CO2) |
| 📁 NH3 (Ammonia) | 📁 Hydrogen (Common) |
| 📁 CaCl2 (Calcium Chloride) | 📁 Nitrogen |
| 📁 Chloride | |
| 📁 Calcium | |
| 📁 Single Bonds | |
| 📁 Double Bonds |